Consider the SN2 reaction between 1-bromo-2-methylpropane, a captivating chemical transformation that unveils the intricacies of nucleophilic substitution reactions. This reaction, characterized by its bimolecular nature and stereospecificity, serves as a cornerstone in organic chemistry, providing a foundation for understanding various organic synthesis techniques.
Delving into the SN2 reaction between 1-bromo-2-methylpropane, we embark on a journey that unravels the fundamental principles governing this reaction, its mechanistic intricacies, and its wide-ranging applications in organic synthesis. Prepare to be enthralled as we uncover the fascinating world of SN2 reactions.
Introduction
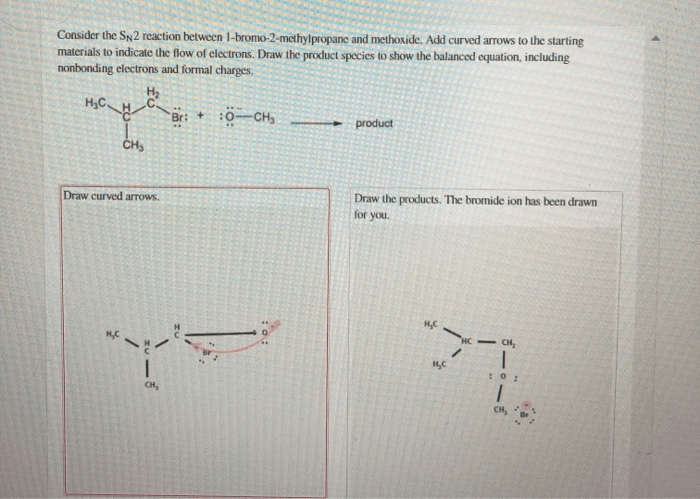
The SN2 reaction, also known as the nucleophilic substitution reaction, is a fundamental reaction in organic chemistry that involves the substitution of a leaving group on an electrophile by a nucleophile. This reaction is characterized by its second-order kinetics, which means that the rate of the reaction is dependent on both the concentration of the nucleophile and the electrophile.
In this article, we will discuss the SN2 reaction between 1-bromo-2-methylpropane and a nucleophile. We will explore the mechanism of the reaction, its stereochemistry, the factors that affect its rate, and its applications in organic synthesis.
Mechanism of the SN2 Reaction
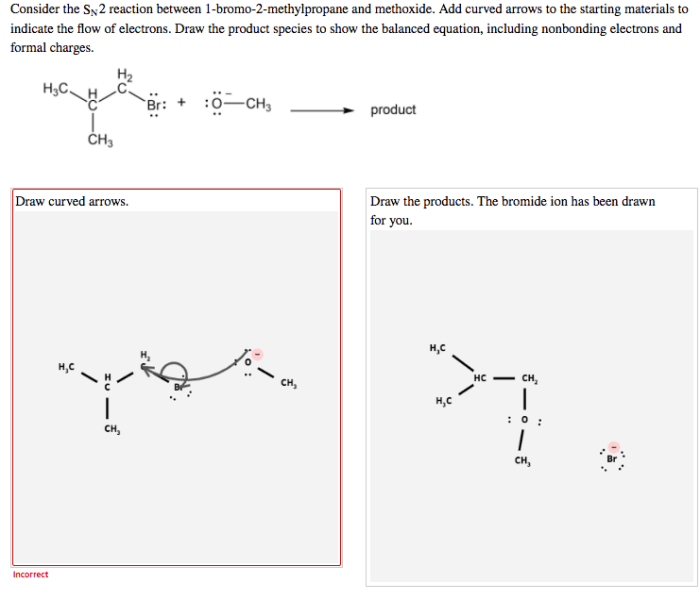
The SN2 reaction between 1-bromo-2-methylpropane and a nucleophile proceeds through a single-step mechanism. The nucleophile attacks the electrophile at the carbon atom bearing the leaving group, and the leaving group is expelled in a concerted fashion.
The transition state of the reaction is a five-membered ring, in which the nucleophile, the electrophile, and the leaving group are all bonded to the same carbon atom. The nucleophile and the leaving group are positioned opposite each other, and the carbon atom is in a trigonal bipyramidal geometry.
| Nucleophile | Electrophile | Leaving Group | Transition State |
|---|---|---|---|
| OH– | (CH3)2CBr | Br– | [(CH3)2CBr…OH–…Br–]– |
Stereochemistry of the SN2 Reaction
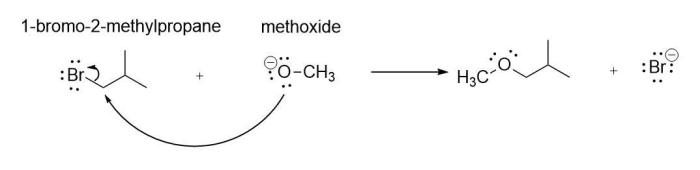
The SN2 reaction is a stereospecific reaction, which means that the stereochemistry of the product is determined by the stereochemistry of the starting materials. In the case of the SN2 reaction between 1-bromo-2-methylpropane and a nucleophile, the reaction proceeds with inversion of configuration at the carbon atom bearing the leaving group.
This inversion of configuration is due to the fact that the nucleophile attacks the electrophile from the back side, opposite to the leaving group. This results in the formation of a product with the opposite stereochemistry at the carbon atom bearing the leaving group.
Factors Affecting the Rate of the SN2 Reaction
The rate of the SN2 reaction between 1-bromo-2-methylpropane and a nucleophile is affected by a number of factors, including:
- Nucleophile strength:The stronger the nucleophile, the faster the reaction will proceed. This is because a stronger nucleophile is more likely to attack the electrophile and displace the leaving group.
- Electrophile strength:The weaker the electrophile, the faster the reaction will proceed. This is because a weaker electrophile is more likely to be attacked by the nucleophile.
- Solvent polarity:The polarity of the solvent can also affect the rate of the SN2 reaction. A polar solvent will stabilize the transition state of the reaction, which will lead to a faster reaction rate.
| Factor | Effect on Rate |
|---|---|
| Nucleophile strength | Increased nucleophile strength leads to increased rate |
| Electrophile strength | Increased electrophile strength leads to decreased rate |
| Solvent polarity | Increased solvent polarity leads to increased rate |
Applications of the SN2 Reaction: Consider The Sn2 Reaction Between 1-bromo-2-methylpropane
The SN2 reaction is a versatile reaction that can be used to synthesize a wide variety of organic compounds. Some of the most common applications of the SN2 reaction include:
- Alkylation of alcohols:The SN2 reaction can be used to alkylate alcohols to form ethers. This reaction is typically carried out using an alkyl halide as the electrophile and an alcohol as the nucleophile.
- Acylation of amines:The SN2 reaction can be used to acylate amines to form amides. This reaction is typically carried out using an acid chloride as the electrophile and an amine as the nucleophile.
- Substitution of halides:The SN2 reaction can be used to substitute halides on alkyl halides with other functional groups. This reaction is typically carried out using a nucleophile that is stronger than the halide ion.
Question & Answer Hub
What is the rate law for the SN2 reaction between 1-bromo-2-methylpropane?
The rate law for the SN2 reaction between 1-bromo-2-methylpropane is: rate = k[nucleophile][electrophile], where k is the rate constant.
What is the stereochemistry of the SN2 reaction between 1-bromo-2-methylpropane?
The SN2 reaction between 1-bromo-2-methylpropane proceeds with inversion of configuration, meaning that the product has the opposite stereochemistry at the carbon atom that undergoes substitution.
What are the factors that affect the rate of the SN2 reaction between 1-bromo-2-methylpropane?
The rate of the SN2 reaction between 1-bromo-2-methylpropane is affected by several factors, including the nucleophilicity of the nucleophile, the electrophilicity of the electrophile, and the polarity of the solvent.