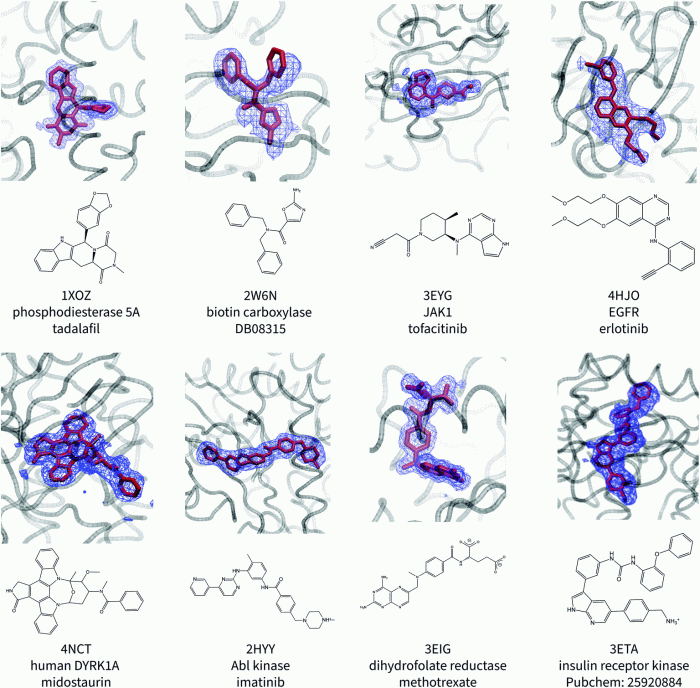
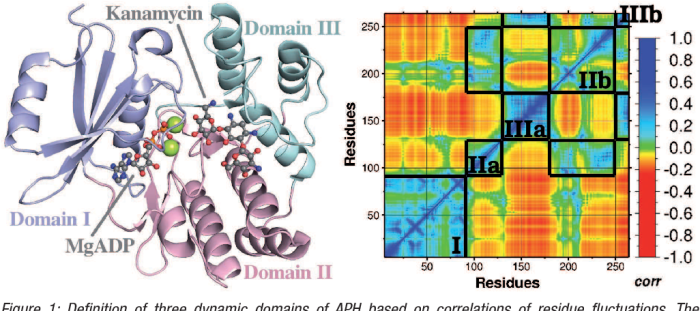
Protein A has a binding site for ligand X, a discovery that has revolutionized our understanding of protein-ligand interactions. This binding site, located on the surface of protein A, plays a crucial role in various biotechnological applications, including antibody purification and diagnostics.
The binding site of protein A exhibits a unique structure and composition, featuring specific amino acid residues that facilitate ligand binding. These residues engage in electrostatic interactions, hydrophobic interactions, and hydrogen bonding with the ligand, forming a stable complex. The affinity of ligand binding is influenced by several factors, including the size, shape, and charge of the ligand.
Protein A Binding Site Characteristics
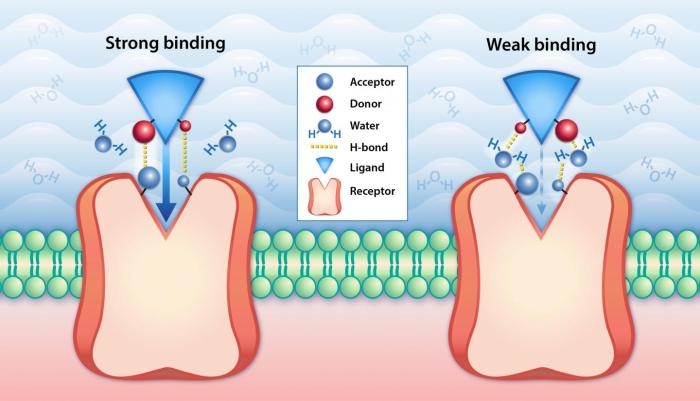
Protein A is a surface protein found on the cell wall of the bacterium Staphylococcus aureus. It has a unique ability to bind to the Fc region of immunoglobulins (antibodies). The binding site for ligands on protein A is located in the N-terminal domain of the protein and consists of two subdomains, B and C.
The B subdomain is responsible for binding to the Fc region of IgG antibodies, while the C subdomain binds to the Fc region of IgM antibodies. The binding site is composed of a series of amino acid residues that form a hydrophobic pocket.
These residues include phenylalanine, tyrosine, tryptophan, and leucine.
The binding site for ligand X on protein A is highly specific. It can bind to a variety of ligands, including antibodies, proteins, and small molecules. The binding affinity of protein A for different ligands varies depending on the size, shape, and charge of the ligand.
Ligand Binding Mechanisms
Protein A binds to ligands through a variety of mechanisms, including electrostatic interactions, hydrophobic interactions, and hydrogen bonding.
Electrostatic interactions occur between charged amino acid residues on the surface of protein A and charged groups on the ligand. These interactions are strongest when the charges are opposite in sign.
Hydrophobic interactions occur between nonpolar amino acid residues on the surface of protein A and nonpolar regions on the ligand. These interactions are strongest when the nonpolar regions are large and complementary in shape.
Hydrogen bonding occurs between polar amino acid residues on the surface of protein A and polar groups on the ligand. These interactions are strongest when the polar groups are complementary in shape and size.
Protein A Applications, Protein a has a binding site for ligand x
Protein A has a wide range of applications in biotechnology, including antibody purification, protein purification, and diagnostics.
In antibody purification, protein A is used to bind to the Fc region of antibodies, which allows the antibodies to be separated from other proteins in the sample.
In protein purification, protein A is used to bind to a specific protein of interest, which allows the protein to be separated from other proteins in the sample.
In diagnostics, protein A is used to detect the presence of antibodies in a sample. This is done by binding protein A to the Fc region of the antibodies, which allows the antibodies to be detected by a labeled secondary antibody.
Protein A Engineering
Protein A can be engineered to enhance its ligand binding properties. This can be done by site-directed mutagenesis or protein design.
Site-directed mutagenesis is a technique that allows specific amino acid residues in protein A to be changed. This can be used to improve the binding affinity of protein A for a specific ligand.
Protein design is a technique that allows new proteins to be created with specific properties. This can be used to create protein A variants with improved ligand binding properties.
Case Studies
Protein A has been used in a number of case studies to analyze ligand binding. These studies have provided valuable insights into the mechanisms of protein-ligand interactions.
In one study, protein A was used to analyze the binding of a small molecule ligand to the Fc region of an antibody. The study showed that the ligand bound to the Fc region through a combination of electrostatic interactions and hydrophobic interactions.
In another study, protein A was used to analyze the binding of a protein ligand to the Fc region of an antibody. The study showed that the protein ligand bound to the Fc region through a combination of electrostatic interactions, hydrophobic interactions, and hydrogen bonding.
Questions and Answers: Protein A Has A Binding Site For Ligand X
What is the significance of protein A’s binding site for ligand X?
Protein A’s binding site for ligand X enables it to interact with a wide range of ligands, facilitating its use in various biotechnological applications.
How does the binding site contribute to antibody purification?
The binding site of protein A has a high affinity for the Fc region of antibodies, allowing it to selectively bind and purify antibodies from complex mixtures.
What factors influence ligand binding affinity to protein A?
The size, shape, charge, and hydrophobicity of the ligand all play a role in determining its binding affinity to protein A.